Abstract
Patients with AML who do not achieve remission with conventional 7+3 induction chemotherapy can frequently achieve CR with an intensive reinduction regimen such as Ida-FLAG. However, when Ida-FLAG is used as the initial induction regimen, the outcome of patients who do not achieve remission and the success of reinduction therapy is unknown.
We identified 100 patients with newly diagnosed AML who received Ida-FLAG as their initial induction chemotherapy between November 2012 and September 2016. The median age was 59 (range 18-77); 49% were male. Twenty had therapy-related AML, 15 had AML following an MPN, 16 following MDS and 10 following an MDS/MPN overlap syndrome; 39 had de novo disease. One had low, 38 intermediate, 53 high risk and 8 unknown/inconclusive cytogenetics. Seventy-two achieved CR/CRi with FLAG-Ida induction. Nine died during induction prior to response assessment, and 19 were refractory. Survival, censored at the time of allogeneic transplantation, was longer for patients who achieved CR/CRi compared to those who did not (median OS 388 days vs 153 days, two-tailed log-rank p<0.001).
Forty-four patients had DNA sequencing for mutations in 54 genes commonly mutated in AML using the TruSight Myeloid Sequencing Panel (TMSP; Illumina, San Diego, CA) on the Illumina MiSeq platform at the time of diagnosis. TP53 mutations were associated with disease refractoriness (p<0.05, corrected for multiple comparisons), as were high risk cytogenetics (p<0.05).
Of the 19 patients whose disease was refractory to initial induction with Ida-FLAG, nine received intensive re-induction chemotherapy with NOVE-HiDAC (n=7), Ida-FLAG (n=1) and HiDAC, mitoxantrone, asparaginase, ATRA, vinblastine, valproate and imatinib (n=1). The remaining 10 patients received a low intensity experimental therapy on a clinical trial (n=1), low intensity/palliative therapy (n=2) or supportive care alone (n=7). Age, gender, presence of secondary AML and cytogenetic risk did not differ between those who did/did not receive reinduction.
Of the 9 patients who received reinduction chemotherapy, only 1 achieved CR. This patient had secondary AML with high-risk cytogenetics and 70% blasts in the marrow at diagnosis. Following initial induction with Ida-FLAG, the patient had 6-7% marrow blasts with no recovery of their peripheral blood counts. The patient received a second course of Ida-FLAG as reinduction and achieved CR 51 days after the start of reinduction chemotherapy. The patient received an allotransplant but died of GVHD 69 days post-transplant. No other patient receiving reinduction chemotherapy responded and none achieved a PR/prolonged aplasia. No patients died during reinduction. No patients receiving experimental agents or low intensity therapy achieved CR or PR. Median OS for those receiving reinduction was similar to patients treated with palliative chemotherapy or clinical trial (182 v 153 days, p=0.2).
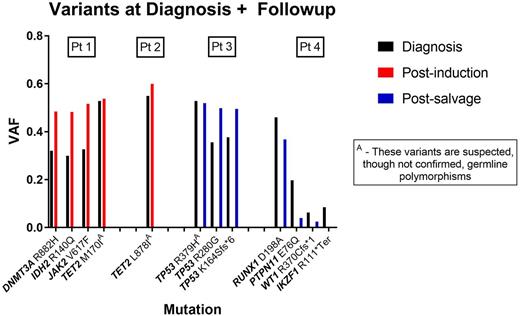
We next assessed how Ida-FLAG and the reinduction regimens affected the genetic clones and subclones in the patients who did not achieve remission with Ida-FLAG. Paired blood or marrow samples from diagnosis and post-treatment were available for 4 consenting patients who did not achieve remission with Ida-FLAG or reinduction. For 2 patients, the second sample was available after Ida-FLAG induction and for 2 patients the second sample was available after reinduction. DNA was extracted and sequenced using the TMSP. All 4 patients had variants identified at diagnosis; 3 patients had recognized relevant somatic variants. All the predominant variants found at diagnosis were detected at follow-up. The variant allele fraction (VAF) of the predominant variants from diagnosis was similar or higher at the time of followup as shown in the figure. In one sample, variants found at low level in the diagnostic sample were reduced following reinduction but the dominant variant remained. No new variants were detected in follow-up samples.
Thus, patients who do not achieve remission with Ida-FLAG as an initial induction regimen have a poor outcome and very low probability of achieving remission with a conventional reinduction regimen. Inferring clonal architecture by using VAF, reinduction chemotherapy may eliminate minor genetic subclones but the dominant clone persists. New therapeutic agents are needed for these patients.
Kamel-Reid: BMS: Research Funding. Gupta: Incyte: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Schimmer: Medivir: Research Funding; Takeda Pharmaceuticals: Research Funding; Novartis Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal